Emissions
Mercury becomes a problem for the environment when it is released from rock and ends up in the atmosphere and in water. These releases can happen naturally. Both volcanoes and forest fires send mercury into the atmosphere.


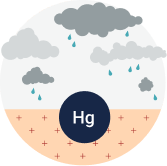
Mercury becomes a problem for the environment when it is released from rock and ends up in the atmosphere and in water. These releases can happen naturally. Both volcanoes and forest fires send mercury into the atmosphere.
Human activities, however, are responsible for much of the mercury that is released into the environment. The burning of coal, oil and wood as fuel can cause mercury to become airborne, as can burning wastes that contain mercury.
This airborne mercury can fall to the ground in raindrops, in dust, or simply due to gravity (known as “air deposition”). The amount of mercury deposited in a given area depends on how much mercury is released from local, regional, national, and international sources.
The main way that people are exposed to mercury is by eating fish and shellfish that have high levels of methylmercury, a highly toxic form of mercury, in their tissues. A less common way people are exposed to mercury is breathing mercury vapor. This can happen when mercury is released from a container, or from a product or device that breaks. If the mercury is not immediately contained or cleaned up, it can evaporate, becoming an invisible, odorless, toxic vapor.
Birds and mammals that eat fish are have more exposures to methylmercury than other animals in water ecosystems. Predators that eat these birds and mammals are also at risk. Methylmercury has been found in eagles, otters, and endangered Florida panthers. At high levels of exposure, methylmercury’s harmful effects on these animals include:
The Minamata Convention on Mercury is a global treaty to protect human health and the environment from the adverse effects of mercury. It was agreed at the fifth session of the Intergovernmental Negotiating Committee on mercury in Geneva, Switzerland at 7 a.m. on the morning of Saturday, 19 January 2013 and adopted later that year on 10 October 2013 at a Diplomatic Conference (Conference of Plenipotentiaries), held in Kumamoto, Japan.
The Minamata Convention entered into force on 16 August 2017, on the 90th day after the date of deposit of the 50th instrument of ratification, acceptance, approval or accession.
The Convention draws attention to a global and ubiquitous metal that, while naturally occurring, has broad uses in everyday objects and is released to the atmosphere, soil and water from a variety of sources. Controlling the anthropogenic releases of mercury throughout its lifecycle has been a key factor in shaping the obligations under the Convention.
Major highlights of the Minamata Convention include a ban on new mercury mines, the phase-out of existing ones, the phase out and phase down of mercury use in a number of products and processes, control measures on emissions to air and on releases to land and water, and the regulation of the informal sector of artisanal and small-scale gold mining.
The Convention also addresses interim storage of mercury and its disposal once it becomes waste, sites contaminated by mercury as well as health issues.
